
Pre-built templates in Smartsheet help you track, manage, and report on clinical research and trial information by improving collaboration, ensuring secure data, and increasing visibility.

Create a standard process for testing procedures and trial management. Smartsheet is designed to unleash the benefits of greater work agility and collaboration by providing a powerful platform for organizations to plan, capture, manage, automate, and report on work.
Break down complex project timelines into manageable tasks and organize them by project phase.
Easily drag and drop Gantt bars to adjust your timeline. Underlying dates automatically adjust.
Set durations for each task and then assign dependencies to ensure proper order of completion.
Track conversations right in your Gantt chart to ensure everyone is on the same page.
Automatically notify team members and stakeholders of upcoming due dates and milestones.
Highlight critical path with the click of a button, so you know which tasks must be completed on time.
Share your project with internal and external stakeholders to ensure visibility.
Attach files and key documentation right to your Gantt chart project plan to keep details in context.
Identify milestones on your Gantt chart to provide a view into progress and ensure you hit important dates.
Set clear expectations and increase accountability by assigning tasks to individual team members.
Import existing work from Excel, MS Project, Google, and Trello, and, export to PDF and PNG files.
Work how you want to by easily switching between grid, Gantt, calendar, and Kanban card views.
Provide stakeholders with a high-level view of key metrics and progress on real-time dashboards.
Use the Smartsheet mobile app for Android and iOS devices to access and manage work from anywhere, at any time.
Keep work connected with integrations in Smartsheet, like Google, O365, Dropbox, Salesforce, Jira, and more.

Learn more about our HIPAA Smartsheet Compliance
Securely share, store, and access PHI while meeting or exceeding all of HIPAA’s regulatory requirements
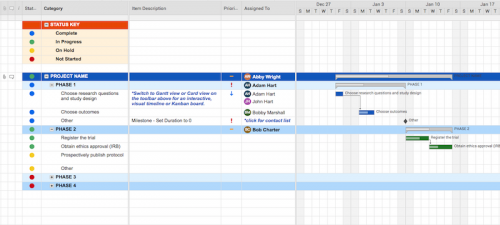
Use this template to develop your own clinical trial timeline. Add your own steps, milestones, and dates for a comprehensive, expansive view.

A project activity list is a detailed, itemized documentation of all the activities scheduled as part of the research project.
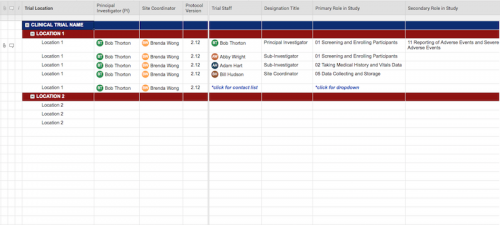
Once roles and responsibilities are determined, the delegation of authority log should be filled out and signed prior to the study’s start.
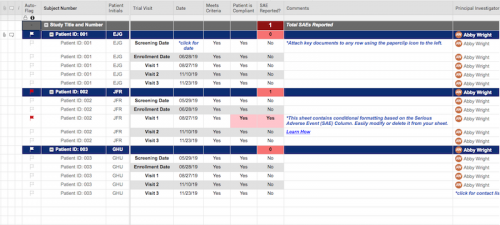
This log keeps track of everyone that has been enrolled for participation in your study, as part of the screening process.

Use a training log to record all training that the site study staff completes, signing the log entry for verification.
![]()
A clinical trial document tracking log can help you keep a written trail of the documents and when they were submitted and approved.

Smartsheet not only allows me to visualize the entire program, but it gave me a platform to optimize my company’s functionality in a way I never knew possible.
Oscar Gonzolez, Program Planning Manager, Karyopharm
Talk with our team to discover how Smartsheet can help modernize and improve your clinical trial operations.